The reaction A + 3B D + F was studied, and the following mechanism was determined 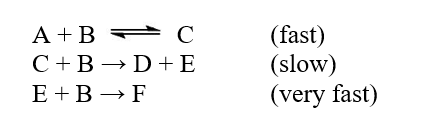 The step with largest activation energy is
The step with largest activation energy is
A) the first step.
B) the second step.
C) the third step.
D) None of the steps has an activation energy.
E) All of the steps have the same activation energy.
Correct Answer:
Verified
Q75: For a one-step reaction, the activation energy
Q76: The reaction progress for a given reaction
Q77: The reaction progress for a given reaction
Q78: The reaction progress for a given reaction
Q79: The reaction progress for a given reaction
Q81: For a given chemical reaction, the rate
Q82: For a given chemical reaction, the rate
Q83: The rate constant for a certain chemical
Q84: For a particular chemical reaction, the rate
Q85: The activation energy for a reaction can
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents