The following questions refer to the phase diagram below. 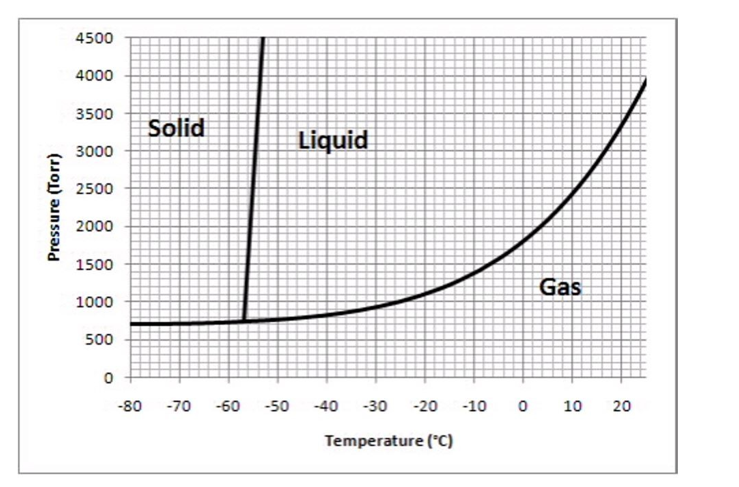
-What phase should this substance exist as, at a pressure of 1500 torr and a temperature of -20°C?
A) solid
B) liquid
C) gas
D) supercritical fluid
E) unable to tell
Correct Answer:
Verified
Q57: The triple point of a substance is
Q58: The following questions refer to the phase
Q59: The following questions refer to the phase
Q60: The following questions refer to the phase
Q61: The following questions refer to the phase
Q63: The following questions refer to the phase
Q64: The following questions refer to the phase
Q65: The following questions refer to the phase
Q66: Which compound cannot be liquefied by compression,
Q67: The critical temperature of a substance is
A)always
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents