The following questions refer to the phase diagram below. 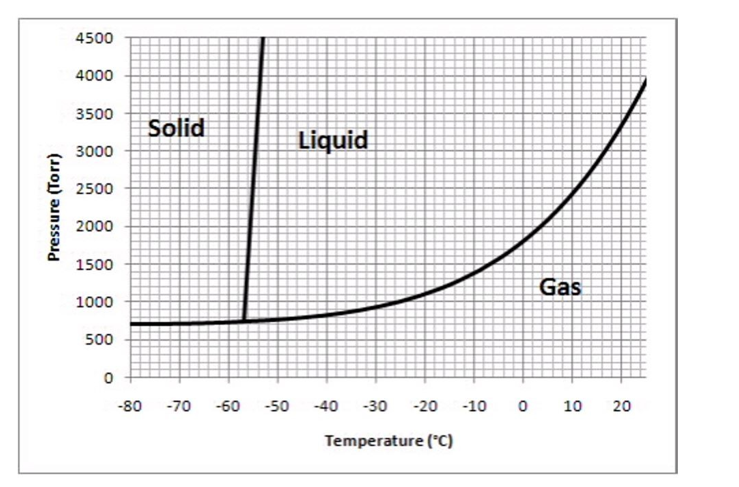
-Which of the following values of temperature and pressure most closely correspond to the triple point of this substance?
A) -21°C and 1000 torr
B) 0°C and 1000 torr.
C) -57°C and 740 torr
D) -50°C and 4500 torr
E) 0°C and 1760 torr
Correct Answer:
Verified
Q60: The following questions refer to the phase
Q61: The following questions refer to the phase
Q62: The following questions refer to the phase
Q63: The following questions refer to the phase
Q64: The following questions refer to the phase
Q66: Which compound cannot be liquefied by compression,
Q67: The critical temperature of a substance is
A)always
Q68: Which compound cannot be liquefied by compression,
Q69: The normal boiling point of acetic acid,
Q70: Find the boiling temperature at 760 torr
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents