The van der Waals equation of state for a real gas is 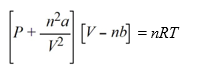 In this equation, the van der Waals constant, b, represents a correction for
In this equation, the van der Waals constant, b, represents a correction for
A) a positive deviation in the measured value of V from that for an ideal gas due to the finite volume of space occupied by molecules of a real gas.
B) a negative deviation in the measured value of V from that for an ideal gas due
To the finite volume of space occupied by molecules of a real gas.
C) a positive deviation in the measured value of V from that for an ideal gas due to the attractive forces between the molecules of a real gas.
D) a negative deviation in the measured value of V from that for an ideal gas due to the attractive forces between the molecules of a real gas.
E) a positive deviation in the measured value of V from that for an ideal gas due to the finite mass of the molecules of a real gas.
Correct Answer:
Verified
Q79: According to the kinetic theory of gases,
Q80: If container "A"is occupied by 1.00 mole
Q81: If container "A"is occupied by 1.00 mole
Q82: Which of the following is a condition
Q83: The van der Waals equation of state
Q85: For a substance that remains a gas
Q86: The van der Waals equation of state
Q87: The van der Waals equation of state
Q88: Air pressure at the top of a
Q89: A Torricelli barometer containing mercury is placed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents