The van der Waals equation of state for a real gas is 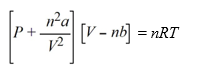 At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
At what pressure will 1.00 mole of NH3 be in a 10.00 L container at 298 K, assuming NH3 is a real gas?(van der Waals constants for NH3 are a = 4.170 L2 atm mol-2, b = 0.03707 L mol-1)
A) 2.03 atm
B) 20.3 atm
C) 2.41 atm
D) 24.1 atm
E) 2.47 atm
Correct Answer:
Verified
Q82: Which of the following is a condition
Q83: The van der Waals equation of state
Q84: The van der Waals equation of state
Q85: For a substance that remains a gas
Q86: The van der Waals equation of state
Q88: Air pressure at the top of a
Q89: A Torricelli barometer containing mercury is placed
Q90: A certain gas is applying a force
Q91: What would a pressure reading of 565
Q92: A gas sample is attached to a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents