What is the energy required to excite a hydrogen atom by causing an electronic transition from the n = 2 to the n = 5 energy level? Recall that the quantized energies of the levels in the hydrogen atom are given by: 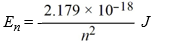
Correct Answer:
Verified
Q135: What is the energy, in joules, of
Q136: In the Rydberg equation, if n1 =
Q137: What is the wavelength of light emitted
Q138: The lowest energy state of an atom
Q139: When an atom absorbs energy it moves
Q141: A wave in which the crests and
Q142: Calculate the wavelength, in meters, of an
Q143: Calculate the wavelength, in meters, of a
Q144: The maximum number of electrons in an
Q145: The maximum number of electrons in an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents