The maximum number of electrons in an atom that can have the following exact same set of quantum numbers is ________. 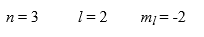
Correct Answer:
Verified
Q140: What is the energy required to excite
Q141: A wave in which the crests and
Q142: Calculate the wavelength, in meters, of an
Q143: Calculate the wavelength, in meters, of a
Q144: The maximum number of electrons in an
Q146: The maximum number of electrons in an
Q147: The number of orbitals in a subshell
Q148: The number of orbitals in a d
Q149: An electron in an atom can be
Q150: A particular energy level in an atom
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents