A volume of 500.0 mL of 0.220 M HCl(aq)was added to a high quality constant-pressure calorimeter containing 500.0 mL of 0.200 M NaOH(aq). Both solutions have a density of and a specific heat of 4.184 J g−1 °C−1. The calorimeter had a heat capacity of 850.0 J °C−1. The temperature of the entire system rose from 25.60 °C to 26.70 °C. Calculate the heat of reaction, in kJ, per mole of NaOH(aq). 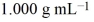 Hint: Consider your reaction taking place and pay careful attention to your mole values.
Hint: Consider your reaction taking place and pay careful attention to your mole values.
Correct Answer:
Verified
Q164: At elevated temperatures, any volatile substances in
Q165: When the rate at which a chemical
Q166: A large oceangoing container vessel (200,000 tons)was
Q167: Explain how it is possible for a
Q168: A constant pressure calorimeter has metal parts
Q170: Propane is often used to heat homes.
Q171: A volume of 600.0 mL of 0.240
Q172: The formulas of ethylene, water, and
Q173: Some workers in central research were talking
Q174: A check of the formulas of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents