The formulas of ethylene, water, and ethanol suggests that the reaction,C2H4(g) + H2O(g) C2H5OH(l) could be made to occur under the correct conditions. This is a wild idea, but Mike says it's just a matter of the right catalyst combination, reactor temperature, and pressure. Using the values in the table below, what is the calculated value of ?H° for Mike's proposed reaction? 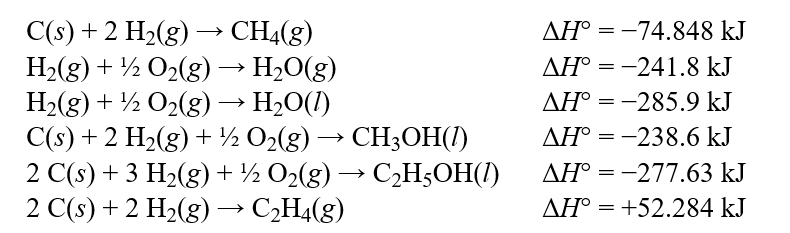 Hint: Be sure to adjust your signs whenever you reverse a reaction.
Hint: Be sure to adjust your signs whenever you reverse a reaction.
A) -39.0 kJ
B) -44.0 kJ
C) +44.0 kJ
D) -88.1 kJ
E) +88.1 kJ
Correct Answer:
Verified
Q166: A large oceangoing container vessel (200,000 tons)was
Q167: Explain how it is possible for a
Q168: A constant pressure calorimeter has metal parts
Q169: A volume of 500.0 mL of 0.220
Q170: Propane is often used to heat homes.
Q171: A volume of 600.0 mL of 0.240
Q173: Some workers in central research were talking
Q174: A check of the formulas of
Q175: The standard enthalpy of combustion for oxalic
Q176: The standard enthalpy of combustion for ethylene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents