For the working galvanic cell shown at standard conditions, how would you increase the cell potential?(If needed, refer to Table 17-1 in the text ) 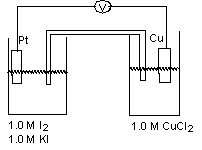
A) Make the Pt electrode larger.
B) Make the Copper electrode larger.
C) Increase the concentration of KI.
D) Increase the concentration of I2.
E) Make the Cu electrode smaller.
Correct Answer:
Verified
Q24: Consult a table of reduction potentials (Table
Q25: Platinum metal is quite resistant to
Q26: Consider an electrochemical cell consisting of
Q27: Consider the Daniell cell where the
Q28: What is the correct description, in
Q30: For the following galvanic cell what will
Q31: Ships, storage tanks, and other large metal
Q32: What are the possible oxidation states of
Q33: How is aluminium protected from oxidation?(If needed,
Q34: Which of the following combinations would provide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents