For the following galvanic cell what will be its potential when the reaction reaches equilibrium?(If needed, refer to Table 17-1in the text ) 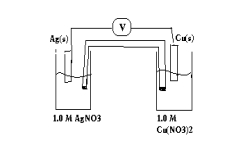
A) 0.0 V
B) 0.458 V
C) 1.142 V
D) 0.272 V
E) 1.26 V
Correct Answer:
Verified
Q25: Platinum metal is quite resistant to
Q26: Consider an electrochemical cell consisting of
Q27: Consider the Daniell cell where the
Q28: What is the correct description, in
Q29: For the working galvanic cell shown at
Q31: Ships, storage tanks, and other large metal
Q32: What are the possible oxidation states of
Q33: How is aluminium protected from oxidation?(If needed,
Q34: Which of the following combinations would provide
Q35: You have an abundant supply of NaCl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents