Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the rate of increase of Cr3+ concentration expressed in terms of changing H3O+ concentration?
A) 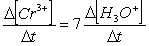
B) 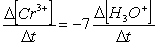
C) 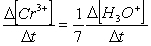
D) 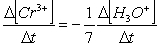
Correct Answer:
Verified
Q12: You are running late for a basketball
Q13: Which of the following sketches shows the
Q14: The synthesis of nitrogen monoxide proceeds
Q15: Consider the aqueous phase reaction between
Q16: Consider the aqueous phase reaction between
Q18: Cyclohexane is manufactured from the reaction
Q19: Cyclohexane is manufactured from the reaction
Q20: NO2 decomposes to form NO and O2.
Q21: Why does the rate of a reaction
Q22: The rate of a reaction
A) increases with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents