Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the reaction rate expressed in terms of changing H3O+ concentration?
A) Reaction rate = 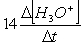
B) Reaction rate = - 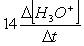
C) Reaction rate = - 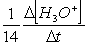
D) Reaction rate = 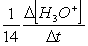
Correct Answer:
Verified
Q10: The formation of chlorocarbon solvents such
Q11: It has been suggested that the
Q12: You are running late for a basketball
Q13: Which of the following sketches shows the
Q14: The synthesis of nitrogen monoxide proceeds
Q16: Consider the aqueous phase reaction between
Q17: Consider the aqueous phase reaction between
Q18: Cyclohexane is manufactured from the reaction
Q19: Cyclohexane is manufactured from the reaction
Q20: NO2 decomposes to form NO and O2.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents