Use the following equation for questions
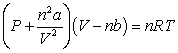
-The relative size of the Van der Waals constant, , correlates well with boiling point; that is, the larger is, the higher the boiling point. The reason for this correlation is
A) the constant is a measure of molecular size.
B) the boiling point is directly proportional to .
C) the constant varies with temperature.
D) the constant is a measure of intermolecular force strength.
E) the constant varies with vapour pressure.
Correct Answer:
Verified
Q5: Understand amorphous and crystalline solids at the
Q6: Explain enthalpies of phase changes in terms
Q7: Which of the following experience the strongest
Q8: Which of the following is a liquid
Q9: Which of the following experience the strongest
Q11: Use the following equation for questions
Q12: Where would you expect Ne to appear
Q13: Which of the following is the expected
Q14: Which is the most realistic picture for
Q15: Consider the following three molecules: 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents