Use the following equation for questions
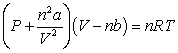
-The value "nb" that is used in the Van der Waals equation accounts for what INCORRECT assumption?
A) that gaseous collisions are not completely elastic
B) that gas molecules do take up space
C) that gas molecules interact with each other
D) that gas molecules do not travel in straight lines
E) that the velocity of gas molecules changes with temperature
Correct Answer:
Verified
Q6: Explain enthalpies of phase changes in terms
Q7: Which of the following experience the strongest
Q8: Which of the following is a liquid
Q9: Which of the following experience the strongest
Q10: Use the following equation for questions
Q12: Where would you expect Ne to appear
Q13: Which of the following is the expected
Q14: Which is the most realistic picture for
Q15: Consider the following three molecules: 
Q16: Which is the expected order of increasing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents