30 In the simple cubic crystal structure at right, the unit cell is outlined in heavy lines which intersect to form the corners at the center of the spheres. If the corners of the unit cells are at the center of the spheres, how many atoms are in one unit cell? 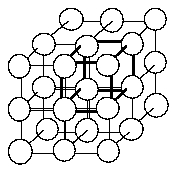
A) 1
B) 2
C) 3
D) 4
E) 6
Correct Answer:
Verified
Q31: For the following substances, choose the answer
Q32: Your solid is non-conductive and melts at
Q33: You have a non-conductive solid. On melting
Q34: You have a solid that it characterized
Q35: Metals are ductile (can be drawn into
Q37: The face-centered cubic unit cell to the
Q38: Ruby is a crystalline compound that contains
Q39: Which type of solid is the most
Q40: Below is the structure for zinc sulphide.
Q41: Polonium metal crystallizes in a simple cubic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents