The face-centered cubic unit cell to the right has been shaded to add clarity; the balls in the corners are black: those in the faces are gray. If the corners of the unit cells are at the center of the corner spheres, how many atoms are in one unit cell? 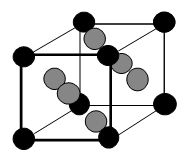
A) 1
B) 2
C) 3
D) 4
E) 6
Correct Answer:
Verified
Q32: Your solid is non-conductive and melts at
Q33: You have a non-conductive solid. On melting
Q34: You have a solid that it characterized
Q35: Metals are ductile (can be drawn into
Q36: 30 In the simple cubic crystal structure
Q38: Ruby is a crystalline compound that contains
Q39: Which type of solid is the most
Q40: Below is the structure for zinc sulphide.
Q41: Polonium metal crystallizes in a simple cubic
Q42: Na+ has an ionic radius of 116
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents