The most energetic electron in any atom at the end of a period of the periodic table is in:
A) an 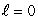 state
state
B) an 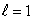 state
state
C) an 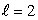 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
Correct Answer:
Verified
Q10: The Pauli exclusion principle is obeyed by:
A)
Q11: No state in an atom can be
Q12: For any atom other that hydrogen and
Q13: The states being filled from the beginning
Q14: The most energetic electron in any atom
Q16: The group of atoms at the ends
Q17: The group of atoms at the beginning
Q18: Suppose the energy required to ionize an
Q19: The effective charge acting on a single
Q20: A laser must be pumped to achieve:
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents