Use the rate laws given below to determine which of the following reactions most likely occurs in a single step.
A) 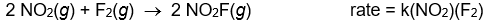
B) 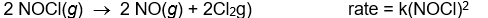
C) 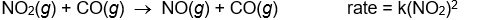
D) 
E) 
Correct Answer:
Verified
Q16: Which of the following affect whether a
Q17: Sum the following elementary steps in
Q18: The reaction between chloroform (CHCl3) and
Q19: The overall rate of a chemical
Q20: Which of the following rate laws suggests
Q22: refer to the reaction:
NO(g) + O3(g)
Q23: refer to the reaction:
NO(g) + O3(g)
Q24: refer to the reaction:
NO(g) + O3(g)
Q25: This data applies to the next three
Q26: This data applies to the next three
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents