This data applies to the next three problems.
The initial rate of disappearance of bromine (Br2) for the reaction shown below was measured for several different concentrations of bromine, CH3COCH3, and H+ ions. (Note that H+ is a catalyst in this reaction - it participates in the reaction but is not itself consumed)
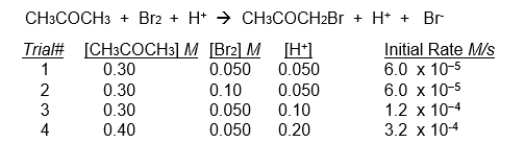
-The rate law for this reaction would be:
A) zero-order in H+ and first-order in Br2
B) first-order in H+ and first-order in Br2
C) first-order in H+ and zero-order in Br2
D) second-order in H+ and zero-order in Br2
E) none of the above.
Correct Answer:
Verified
Q20: Which of the following rate laws suggests
Q21: Use the rate laws given below to
Q22: refer to the reaction:
NO(g) + O3(g)
Q23: refer to the reaction:
NO(g) + O3(g)
Q24: refer to the reaction:
NO(g) + O3(g)
Q26: This data applies to the next three
Q27: This data applies to the next three
Q28: The reaction shown below is first
Q29: The reaction following reaction was studied at
Q30: Use the following data on the initial
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents