Use the following data to answer
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
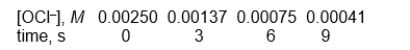
-What is the rate constant (k) for this reaction?
A) between 10-2 and 10-1
B) larger than 10-1 and smaller than 1
C) between 1 and 10
D) larger than 10 and smaller than 100
E) between 100 and 1000
Correct Answer:
Verified
Q54: The reaction, N2O5
Q55: The following data table should be
Q56: The following data table should be
Q57: The following data table should be
Q58: If a plot of 1/[A] versus time
Q60: Use the following data to answer
Q61: Use the following data to answer
Q62: refer to the following reaction for
Q63: refer to the following reaction for
Q64: refer to the following reaction for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents