Use the following data to answer
These data were obtained by monitoring the rate at which the OCl- ion was consumed in the presence of a large excess of the I- ion.
OCl-(aq) + I-(aq) OI-(aq) + Cl-(aq)
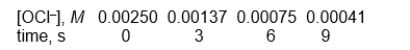
-What is the half-life in seconds?
A) between 0.01 and 0.1 seconds
B) between 0.1 and 1 second
C) between 1 and 10 seconds
D) between 10 and 100 seconds
E) none of the above
Correct Answer:
Verified
Q55: The following data table should be
Q56: The following data table should be
Q57: The following data table should be
Q58: If a plot of 1/[A] versus time
Q59: Use the following data to answer
Q61: Use the following data to answer
Q62: refer to the following reaction for
Q63: refer to the following reaction for
Q64: refer to the following reaction for
Q65: refer to the reaction
2 N2O5(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents