Which statement correctly describes the following reaction? 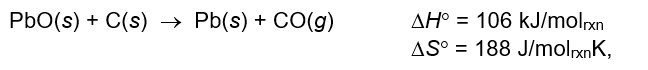
A) The reaction is spontaneous at all temperatures.
B) The reaction is spontaneous only below about 300 K.
C) The reaction becomes spontaneous at about 300 K.
D) The reaction becomes spontaneous at about 560 K.
E) The reaction is spontaneous only above 2000 K.
Correct Answer:
Verified
Q15: Given that
Q16: For which of the following highly
Q17: Which of the following combinations of
Q18: Under which one of the following conditions
Q19: Which of the following combinations of
Q21: The entropy change in the process:
Q22: Which of the following relationships between
Q23: The Kp for the following equation
Q24: Assume that Kp = 1.75 for the
Q25: The composition of the following system at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents