Assume that Kp = 1.75 for the reaction.
CO2(g) + C(s) 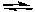 2 CO(g)
2 CO(g)
Calculate Kp for the following reaction.
CO(g)  1/2 CO2(g) + 1/2 C(s)
1/2 CO2(g) + 1/2 C(s)
A) 0.756
B) 1.32
C) 1.75
D) 3.06
E) none of the above
Correct Answer:
Verified
Q19: Which of the following combinations of
Q20: Which statement correctly describes the following reaction?
Q21: The entropy change in the process:
Q22: Which of the following relationships between
Q23: The Kp for the following equation
Q25: The composition of the following system at
Q26: Given the equilibrium constant for the following
Q27: Which statement concerning the following reaction isn't
Q28: The standard free energy change for
Q29: Which of the following statements is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents