Given the equilibrium constant for the following reaction,
H2(g) + Cl2(g) 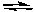 2 HCl(g) Kp = 2.5 x 104
2 HCl(g) Kp = 2.5 x 104
If equimolar amounts of H2 and Cl2 are allowed to reach equilibrium in a system in which no HCl was present initially, the ratio of the partial pressures of H2 to Cl2 at equilibrium will be
A) larger than 1.
B) smaller than 1.
C) equal to 1.
D) a value that can't be generalized by any of the above statements.
Correct Answer:
Verified
Q21: The entropy change in the process:
Q22: Which of the following relationships between
Q23: The Kp for the following equation
Q24: Assume that Kp = 1.75 for the
Q25: The composition of the following system at
Q27: Which statement concerning the following reaction isn't
Q28: The standard free energy change for
Q29: Which of the following statements is
Q30: Which of the following statements is
Q31: If the following reaction is spontaneous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents