Calculate the standard free energy, G ° , in kJ/molrxn for the reaction below.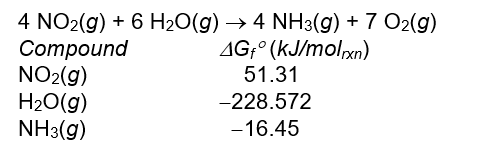
A) -1232.0 kJ/molrxn
B) 1232.0 kJ/molrxn
C) -478.9 kJ/molrxn
D) 478.9 kJ/molrxn
E) none of these
Correct Answer:
Verified
Q38: Which statement is true if the
Q39: What happens to the magnitude of the
Q40: Which of the following statements is
Q41: Although the diatomic molecule N2 has
Q42: Calculate the standard free energy change,
Q44: Calculate the standard enthalpy change,
Q45: For each of the following variables
Q46: In chapter 12 you used Eo
Q47: The equilibrium constant for the following reaction
Q48: The equilibrium constant for the following reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents