Oxidation-reduction reactions are often written with double arrows indicating that the reaction can go in either direction depending on the experimental conditions. For the following reaction identify the oxidizing agent on both sides of the chemical equation.
S2O82-(aq) + Zn(s) 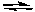 Zn2+(aq) + 2 SO42-(aq)
Zn2+(aq) + 2 SO42-(aq)
A) S2O82- and SO42-
B) Zn and SO42-
C) Zn and Zn2+
D) S2O82- and Zn2+
E) none of these
Correct Answer:
Verified
Q16: Consider the following generalized half-reactions.
Aox + e-
Q17: The potential of a cell at standard
Q18: What is the potential for a voltaic
Q19: What is the standard cell potential
Q20: What is the magnitude of the
Q22: Which statement correctly describes the following
Q23: Which of the following is true
Q24: Identify each of the requested compounds
Q25: Which statement correctly describes the following
Q26: For the following reaction, identify the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents