Consider the following generalized half-reactions.
Aox + e- 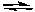 Ared
Ared
Box + e- 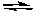 Bred
Bred
If E° for the half-reaction involving A is positive and if E° for the half-reaction involving B is negative, then at standard conditions:
A) Aox will reduce Box
B) Ared will reduce Box
C) Bred will reduce Aox
D) Bred will reduce Ared
E) no reaction will occur
Correct Answer:
Verified
Q11: Which of the following isn't an
Q12: A voltaic cell is constructed with
Q13: What will be the coefficients of
Q14: Use a table of standard reduction potentials
Q15: Use the table of electrode potentials
Q17: The potential of a cell at standard
Q18: What is the potential for a voltaic
Q19: What is the standard cell potential
Q20: What is the magnitude of the
Q21: Oxidation-reduction reactions are often written with double
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents