Which of the following isn't an example of an oxidation-reduction reaction?
A) H2(g) + Cl2(g) 2 HCl(g)
B) Ag+(aq) + 2 NH3(g) 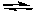 Ag(NH3) 2+(aq)
Ag(NH3) 2+(aq)
C) Hg2Cl2(s) + NH3(g) Hg(s) + HgNH2Cl(s)
D) 2 Mg(s) + O2(g) 2MgO(s)
E) Cl2(g) + 2 Br-(aq) 2 Cl-(aq) + Br2(l)
Correct Answer:
Verified
Q6: Q7: The carbon in bold in the ethanol Q8: Which of the following statements about this Q9: How many electrons are transferred in the Q10: Which of the following isn't an Q12: A voltaic cell is constructed with Q13: What will be the coefficients of Q14: Use a table of standard reduction potentials Q15: Use the table of electrode potentials Q16: Consider the following generalized half-reactions.![]()
Aox + e-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents