Use a table of standard reduction potentials to determine which of the following statements is true for the electrochemical cell diagrammed below. 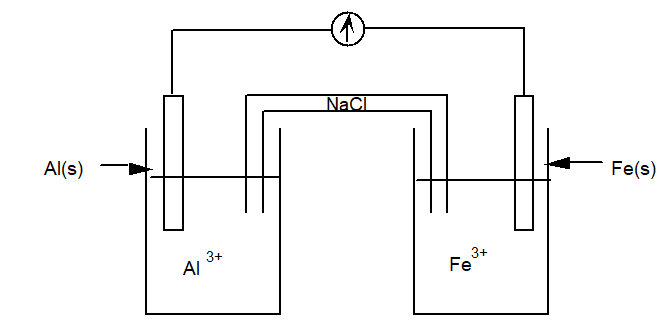
A) The Al is the cathode.
B) Electrons move from the Al electrode to the Fe electrode.
C) The mass of the Al electrode decreases.
D) Both (b) and (c) are true. .
E) Both (a) and (c) are true
Correct Answer:
Verified
Q9: How many electrons are transferred in the
Q10: Which of the following isn't an
Q11: Which of the following isn't an
Q12: A voltaic cell is constructed with
Q13: What will be the coefficients of
Q15: Use the table of electrode potentials
Q16: Consider the following generalized half-reactions.
Aox + e-
Q17: The potential of a cell at standard
Q18: What is the potential for a voltaic
Q19: What is the standard cell potential
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents