Calculate the complex dissociation equilibrium constant (Kd) for the Cd(NH3) 42+ complex from the following data at 298K. 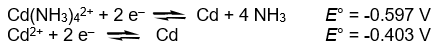
A) 2.7 x 10-7
B) 0.19
C) 6.6
D) 3.6 x 106
E) none of the above
Correct Answer:
Verified
Q40: The equilibrium constant at 298 K
Q41: The equilibrium constant at 25 oC
Q42: Calculate the value of the Ksp for
Q43: There are two half-reactions for the reduction
Q44: The half-reaction reduction potentials for the mercury(I)
Q46: Calculate the weight of sodium metal that
Q47: What is the ratio of the weight
Q48: Calculate the amount of aluminum produced in
Q49: Suppose a beer can weighs 40.0 g.
Q50: Which of the following electrolysis processes will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents