NaHCO3 can be used to neutralize strong bases, such as NaOH. What conclusion can be drawn from the fact that the following acid-base reaction proceeds to the right as written?
HCO3-(aq) + OH-(aq) 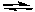 CO32-(aq) + H2O(l)
CO32-(aq) + H2O(l)
A) HCO3- is a stronger acid than H2O.
B) HCO3- is a stronger base than CO32-.
C) HCO3- is a stronger base than OH-.
D) CO32- is a stronger base than OH-.
E) H2O is a stronger acid than HCO3-.
Correct Answer:
Verified
Q79: Which of the following solutions has the
Q80: The addition of sodium formate (HCO2Na) to
Q81: Which of the following would form the
Q82: Which of the following compounds would give
Q83: Which of the following is correct (assume
Q85: What can we conclude from the fact
Q86: Which of the following solutions would have
Q87: Which one of the following mixtures would
Q88: Which of the following solutions will show
Q89: What is the pH of a solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents