What is the pH of a solution that is simultaneously 0.10 M in both NH3 and the NH4+ ion?
(NH4+: Ka = 5.6 x 10-10)
NH3(aq) + H2O(l) 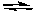 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
A) 4.75
B) 7.00
C) 9.25
D) The pH of this solution would be impossible to predict.
E) none of the above
Correct Answer:
Verified
Q84: NaHCO3 can be used to neutralize strong
Q85: What can we conclude from the fact
Q86: Which of the following solutions would have
Q87: Which one of the following mixtures would
Q88: Which of the following solutions will show
Q90: What would be the effect of doubling
Q91: What is the [HOAc]/[OAc-] ratio in an
Q92: Calculate the pH of a buffer prepared
Q93: What is the pH of a solution
Q94: If equimolar amounts of NH3 and NH4Cl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents