What is the pH of a solution that is 0.01 M in CH3NH2 and 0.1 M in CH3NH3+?
CH3NH2(aq) + H2O(l) 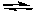 CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
A) 2.36
B) 3.36
C) 9.64
D) 10.64
E) 11.64
Correct Answer:
Verified
Q88: Which of the following solutions will show
Q89: What is the pH of a solution
Q90: What would be the effect of doubling
Q91: What is the [HOAc]/[OAc-] ratio in an
Q92: Calculate the pH of a buffer prepared
Q94: If equimolar amounts of NH3 and NH4Cl
Q95: When NH4Cl is added to a 0.10
Q96: When would the pH of a solution
Q97: Which of the following solutions would be
Q98: What reaction would take place to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents