At what point in the following titration curve would the pH of the solution be equal to the pKa of the acid?
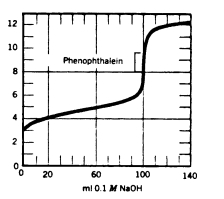
A) 0 mL
B) 50 mL
C) 95 mL
D) 100 mL
E) 120 mL
Correct Answer:
Verified
Q98: What reaction would take place to
Q99: Predict the products of the following acid-base
Q100: Predict the products of the following acid-base
Q101: Which of the following isn't an acid-base
Q102: Which of the following acid-base reactions should
Q104: Which of the following compounds can act
Q105: The dissociation of water into H3O+ and
Q106: Rank the following 0.100 M solutions in
Q107: "Cocaine", as it is shown on television,
Q108: Rank the following in order of increasing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents