What is the pH of a 0.10 M succinic acid, H2Sc, solution if we assume that H2Sc is a weak acid that undergoes stepwise dissociation?
H2Sc(aq) + H2O(l) 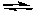 H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l) 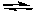 H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
A) 2.1
B) 2.6
C) 3.3
D) 4.2
E) 5.6
Correct Answer:
Verified
Q107: "Cocaine", as it is shown on television,
Q108: Rank the following in order of increasing
Q109: Use the following acid-dissociation equilibrium constants for
Q110: Use the following acid-dissociation equilibrium constants for
Q111: Use the following acid-dissociation equilibrium constants for
Q113: Use the following information to answer
H2Se:
Q114: Use the following information to answer
H2Se:
Q115: Use the following information to answer
H2Se:
Q116: Sulfurous acid (H2SO3) is an acid and
Q117: Succinic acid (HO2CCH2CH2CO2H) is an intermediate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents