KHS can act as a weak acid or a weak base.
HS-(aq) + H2O(l) 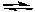 S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
HS-(aq) + H2O(l) 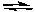 H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
Which of the following statements is correct?
A) A solution of 0.1 M K2S and 0.1 M KHS will be acidic.
B) A solution of 0.01 M H2S will have a pH = 2.
C) A solution of 0.1 M KHS will be weakly basic.
D) H2S is over 90% ionized in water solution.
E) The S2- ion concentration in a 0.1 M KHS solution does not depend on pH.
Correct Answer:
Verified
Q115: Use the following information to answer
H2Se:
Q116: Sulfurous acid (H2SO3) is an acid and
Q117: Succinic acid (HO2CCH2CH2CO2H) is an intermediate
Q118: Using data from the previous problem, what
Q119: What is the pH of a 0.10
Q120: Use the relationship between Ka1 and
Q121: A diprotic acid, H2A, has the following
Q122: For a weak diprotic acid, H2A, for
Q123: Which of the following equations can be
Q124: For H2CO3, Ka2 = 4.7 x 10-11.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents