Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
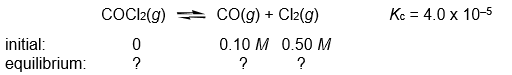
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q30: A 0.100 M sample of SO2 reacts
Q31: Calculate the concentrations of Cl2 and ClF3
Q32: For the reaction Q33: What is the relationship between the changes Q34: For the following reaction Q36: Calculate the NO, NO2, and O2 concentrations Q37: Calculate the equilibrium concentrations of SO3, SO2, Q38: Calculate the concentrations of all reactants and Q39: Assume an equilibrium constant Kc = 1.7 Q40: Calculate the COCl2, CO, and Cl2 concentrations
2 NOCl(g) ![]()
2 C2H6(g) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents