Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
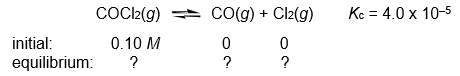
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q35: Calculate the COCl2, CO, and Cl2 concentrations
Q36: Calculate the NO, NO2, and O2 concentrations
Q37: Calculate the equilibrium concentrations of SO3, SO2,
Q38: Calculate the concentrations of all reactants and
Q39: Assume an equilibrium constant Kc = 1.7
Q41: Calculate the NO, NO2, and O2 concentrations
Q42: Hidden Assumptions that make Equilibrium Calculations Easier
Q43: Hidden Assumptions that make Equilibrium Calculations
Q44: Hidden Assumptions that make Equilibrium Calculations Easier
Q45: Hidden Assumptions that make Equilibrium Calculations Easier
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents