Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g)  2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-The equation below solves for the change in concentration for the given reaction assuming the initial concentration of H2O(g) is 0.10 M. Which equation will result if it is assumed that the change will be much smaller than the initial concentration of H2O(g) ?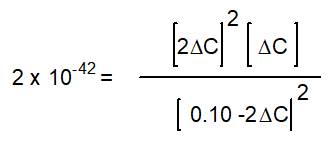
A) 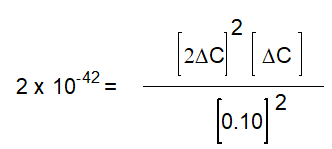
B) 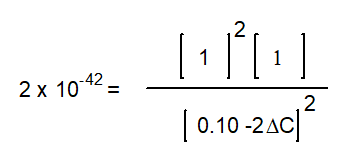
C) 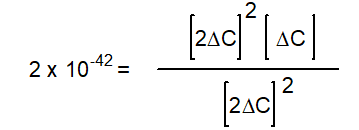
D) 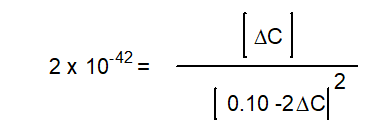
E) None of the above
Correct Answer:
Verified
Q39: Assume an equilibrium constant Kc = 1.7
Q40: Calculate the COCl2, CO, and Cl2 concentrations
Q41: Calculate the NO, NO2, and O2 concentrations
Q42: Hidden Assumptions that make Equilibrium Calculations Easier
Q43: Hidden Assumptions that make Equilibrium Calculations
Q45: Hidden Assumptions that make Equilibrium Calculations Easier
Q46: What would happen if F2 was removed
Q47: What is the effect of increasing the
Q48: What would happen to the extent of
Q49: Which of the following factors will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents