Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 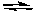 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO) 5 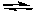 Fe(CO) 4 + CO
Fe(CO) 4 + CO
Initial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
A) to make both Qc and Kc large
B) to make both Qc and Kc small
C) to bring Qc as close as possible to Kc
D) to make the difference between Qc and Kc as large as possible
Correct Answer:
Verified
Q40: Calculate the COCl2, CO, and Cl2 concentrations
Q41: Calculate the NO, NO2, and O2 concentrations
Q42: Hidden Assumptions that make Equilibrium Calculations Easier
Q43: Hidden Assumptions that make Equilibrium Calculations
Q44: Hidden Assumptions that make Equilibrium Calculations Easier
Q46: What would happen if F2 was removed
Q47: What is the effect of increasing the
Q48: What would happen to the extent of
Q49: Which of the following factors will
Q50: The following chemical reaction has reached
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents