The Following Chemical Reaction Has Reached Equilibrium H = -922 KJ/molrxn
A) Increasing the Pressure
B) Increasing the Concentration
The following chemical reaction has reached equilibrium. Which of the changes listed below would cause the equilibrium to shift back toward the reactants?
N2(g) + 3 H2(g) 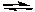 2 NH3(g) H = -92.2 kJ/molrxn
2 NH3(g) H = -92.2 kJ/molrxn
A) increasing the pressure
B) increasing the concentration of N2
C) increasing the temperature
D) decreasing the concentration of NH3
E) none of these
Correct Answer:
Verified
Q45: Hidden Assumptions that make Equilibrium Calculations Easier
Q46: What would happen if F2 was removed
Q47: What is the effect of increasing the
Q48: What would happen to the extent of
Q49: Which of the following factors will
Q51: The following reaction is at equilibrium. Which
Q52: The following reactions are at equilibrium. Beside
Q53: The following reactions are at equilibrium. Which
Q54: What would happen if Cl2 is added
Q55: Which of the following statements correctly
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents