What would happen if Cl2 is added to the following system at equilibrium?
H2(g) + Cl2(g) 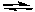 2 HCl(g)
2 HCl(g)
A) The concentrations of both H2 and HCl would remain the same.
B) The concentrations of both H2 and HCl would increase.
C) The concentrations of both H2 and HCl would decrease.
D) The concentration of H2 would increase, but HCl would decrease.
E) The concentration of H2 would decrease, but HCl would increase.
Correct Answer:
Verified
Q49: Which of the following factors will
Q50: The following chemical reaction has reached
Q51: The following reaction is at equilibrium. Which
Q52: The following reactions are at equilibrium. Beside
Q53: The following reactions are at equilibrium. Which
Q55: Which of the following statements correctly
Q56: What would be the effect of removing
Q57: The addition of I2(g) to a fixed
Q58: What would happen if O2 were removed
Q59: The equilibrium constant for the following reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents