What would happen if O2 were removed from the following system at equilibrium at 25°C?
2 NO2(g) 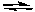 2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
A) The NO2 and NO concentrations would increase.
B) The NO2 and NO concentrations would decrease.
C) The NO2 concentration would increase and the NO concentration would decrease.
D) The NO2 concentration would decrease and the NO concentration would increase.
E) none of the above
Correct Answer:
Verified
Q53: The following reactions are at equilibrium. Which
Q54: What would happen if Cl2 is added
Q55: Which of the following statements correctly
Q56: What would be the effect of removing
Q57: The addition of I2(g) to a fixed
Q59: The equilibrium constant for the following reaction
Q60: What is the effect of increasing the
Q61: What would happen to the extent of
Q62: What would be the effect of decreasing
Q63: One of the products of the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents