Which of the following equilibria would not be affected by changes in pressure?
A) 2 NO(g) + O2(g) 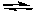 2 NO2(g)
2 NO2(g)
B) N2O4(g) 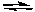 2 NO2(g)
2 NO2(g)
C) 4 NH3(g) + 5 O2(g) 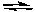 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
D) CaCO3(s) 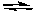 CaO(s) + CO2(g)
CaO(s) + CO2(g)
E) CO(g) + H2O(g) 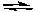 CO2(g) + H2(g)
CO2(g) + H2(g)
Correct Answer:
Verified
Q59: The equilibrium constant for the following reaction
Q60: What is the effect of increasing the
Q61: What would happen to the extent of
Q62: What would be the effect of decreasing
Q63: One of the products of the reaction
Q65: What is the concentration in moles per
Q66: What is the correct solubility constant expression
Q67: Which is the correct reaction for the
Q68: What is the correct solubility constant expression
Q69: What is the correct solubility constant expression
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents