Which is the correct reaction for the solubility constant expression (Ksp) of CaF2(s) ?
A) 2F-(aq) + Ca2+(aq) 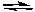 CaF2(s)
CaF2(s)
B) 2F(aq) + Ca (aq) 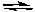 CaF2(s)
CaF2(s)
C) CaF2(s) 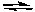 CaF2(g)
CaF2(g)
D) CaF2(s) 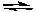 Ca(g) + 2F(g)
Ca(g) + 2F(g)
E) CaF2(s) 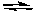 Ca2+(aq) + 2F-(aq)
Ca2+(aq) + 2F-(aq)
Correct Answer:
Verified
Q62: What would be the effect of decreasing
Q63: One of the products of the reaction
Q64: Which of the following equilibria would not
Q65: What is the concentration in moles per
Q66: What is the correct solubility constant expression
Q68: What is the correct solubility constant expression
Q69: What is the correct solubility constant expression
Q70: What is the correct solubility constant expression
Q71: What is the solubility in moles per
Q72: What is the solubility in moles/L of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents