Use the bond-type triangle for
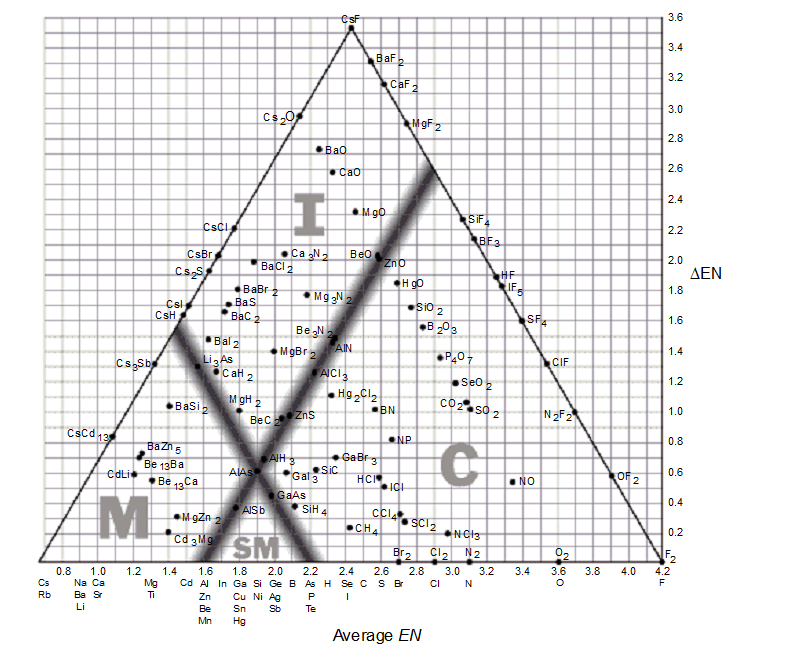
-Predict which compound has a high melting point and is a conductor in the molten and aqueous state.
A) AlSb
B) AlN
C) AlLi
D) AlP
E) AlAs
Correct Answer:
Verified
Q8: A compound that is a poor conductor
Q9: Arrange the following ionic compounds in order
Q10: Use the bond-type triangle for
Q11: Use the bond-type triangle for
Q12: Use the bond-type triangle for
Q14: Use the bond-type triangle for
Q15: Use the bond-type triangle for
Q16: Use the bond-type triangle for
Q17: Which of the following structures has a
Q18: Which of the following correctly describes a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents