What is the relationship between the unit cell edge length (a) and the radius of a metal atom (r) when the metal crystallizes in a face-centered cubic unit cell?
A) r = 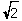 a
a
B) r = ( 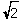 a) /4
a) /4
C) r = ( 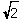 a) /2
a) /2
D) r = ( 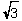 a) /4
a) /4
E) none of the above
Correct Answer:
Verified
Q15: Use the bond-type triangle for
Q16: Use the bond-type triangle for
Q17: Which of the following structures has a
Q18: Which of the following correctly describes a
Q19: What is the ratio of the length
Q21: Calcium titanate crystallizes in a cubic unit
Q22: Calculate the number of chloride and ammonium
Q23: What is the density in g/cm3 of
Q24: What is the approximate density in g/cm3
Q25: Tungsten crystallizes in a cubic unit cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents