The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-How much energy in joules is required to heat 10.0 grams of gold from room temperature (20.0°C) to the temperature of boiling water (100.0°C) if the specific heat of gold is 0.129 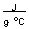 ?
?
A) 1.29 J
B) 10.3 J
C) 103 J
D) 6,200 J
E) none of the above
Correct Answer:
Verified
Q1: The following questions often assume that a
Q2: The following questions often assume that a
Q3: The following questions often assume that a
Q5: The following questions often assume that a
Q6: The following questions often assume that a
Q7: The change in enthalpy,
Q8: Which of the following is a
Q9: For the reaction
MnO2(s) + CO(g)
Q10: How much heat is produced when 0.200
Q11: How much heat in kilojoules is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents