Short Answer
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Given that H ° for this reaction is -5645 kJ/molrxn, what is the value of Hf ° for sucrose?
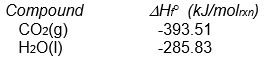
Correct Answer:
Verified
-2221 kJ/m...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents